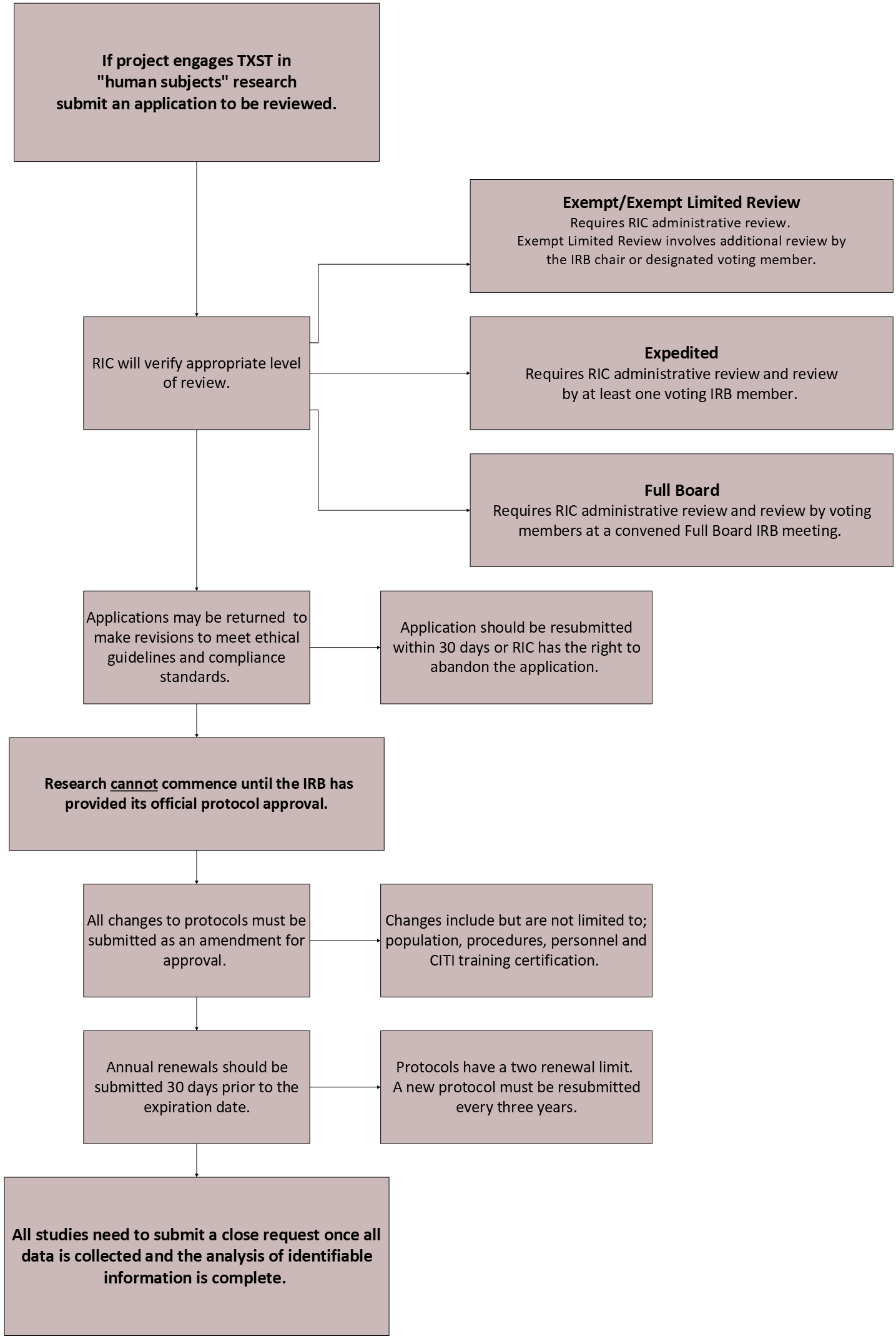
Review Process
Level of Review
-
Exempt
-
Information
Exempt review is applicable for research activities that involve no more than minimal risk to the human participants and fall into one or more of the eight exempt categories defined by the federal regulations. All exempt research must be conducted ethically per the Bellmont Report utilizing equitable selection of participants and a consent process.
-
Categories
-
Category 1 - Educational Exemption
Research conducted in established or commonly accepted educational settings, that specifically involves normal educational practices that are not likely to adversely impact students’ opportunity to learn required educational content or the assessment of educators who provide instruction.
Examples:
- Effectiveness of on-line training as supplement to regular instructional approach
- Evaluation of two currently implemented instructional techniques, curricula, or classroom management methods
-
Category 2 - Surveys, Interviews, Educational Tests, & Observation of Public Behavior
Research only includes interactions involving educational tests, survey procedures, interview procedures, or observation of public behavior. Information must be obtained so that the participant cannot be identified, and the research does not put them at risk in their personal lives including legal issues, employment, or educational advancement.
Examples:
- An online survey that collects anonymous data about factors contributing to student success.
- An interview about living on-campus that does not record any identifying information about the participant.
-
Category 3 - Benign Behavioral Intervention
Research involving benign behavioral interventions that are brief in duration, harmless, painless, not physically invasive, not likely to have a significant adverse lasting impact on the subjects. Information must be obtained so that the participant cannot be identified, and the research does not put them at risk in their personal lives including legal issues, employment, or educational advancement.
Examples:
- Subjects can play a short video game while their physiological stress is measured.
- Subjects can complete an anonymous survey before and after brief exercising to study the effect of physical activity on wellness.
-
Category 4 - Secondary Research (Identifiable Private Information/Biospecimens)
Secondary research use of identifiable private information or identifiable biospecimens for which consent is not required because it is publicly available information.
Examples:
- Patient data extracted from medical records without name or ID number every 6 months as follow up visits occur.
- An analysis of biospecimens from an IRB-approved biorepository.
-
Category 5 - Public Benefit/Service Program Research (Federal Demonstration Projects)
Research supported by a federal agency studying, evaluating, or examining public benefit or service programs. This includes procedures for obtaining benefits or services under those programs, possible changes in or alternatives to those programs or procedures, or possible changes in methods or levels of payment for benefits or services under those programs.
Examples:
- Outcomes assessment from government-sponsored mental health services.
- Study of barriers to obtaining new Medicare benefits.
-
Category 6 - Taste/Food Quality Evaluation & Consumer Acceptance
Research involving taste and food quality evaluation of consumer acceptance studies.
Examples:
- A taste-test on different varieties of fruit that do not have additives to determine consumer preference.
- Study looking at approved levels of an agricultural chemical on taste of vegetables.
-
Category 7 - Storage/Maintenance of Identifiable Biospecimens for secondary research use with Broad Consent
Storage or maintenance of identifiable private information or identifiable biospecimens for secondary research use.
Examples:- Creating a dataset containing identifiers from a previous study to conduct future research.
- Saving blood samples from collaborator’s study for a future research question
-
Category 8 - Secondary data use of Identifiable Data/Biospecimens Obtained with Broad Consent
Secondary research for which broad consent is required: Research involving the use of identifiable private information or identifiable biospecimens for secondary research use.
Examples:
- Using dataset from prior study containing identifiers to answer subsequent research question.
- Using blood samples from collaborator’s study for an additional research question.
-
-
-
Expedited Review
-
Information
Expedited review is applicable for research activities that involve no more than minimal risk to the human participants and fall into one or more of the nine expedited categories defined by the federal regulations.
-
Categories
-
Category 1 - Clinical studies of drugs and medical devices
Clinical studies of drugs or which an investigational new drug application (21 CFR Part 312) is not required and medical devices for which an investigational device exemption application (21 CFR Part 812) is not required or (the medical device is cleared/approved for marketing and the medical device is being used in accordance with its cleared/approved labeling.
-
Category 2 - Collection of blood samples by finger stick, heel stick, ear stick, or venipuncture
Collection of blood samples by finger stick, heel stick, ear stick, or venipuncture from healthy, nonpregnant adults and children, considering the age, weight, and health of the subjects, the collection procedure, the amount of blood to be collected, and the frequency with which it will be collected may not occur more frequently than 2 times per week.
-
Category 3 - Prospective collection of biological specimens for research purposes by noninvasive means
Prospective collection of biological specimens for research purposes by noninvasive means including but not limited to; hair, nails, teeth, external secretions and skin cells
-
Category 4 - Collection of data through noninvasive procedures (not involving general anesthesia or sedation) routinely employed in clinical practice
Collection of data through noninvasive procedures routinely employed in clinical practice, excluding procedures involving x-rays or microwaves. Where medical devices are employed, they must be cleared/approved for marketing.
-
Category 5 - Research involving materials (data, documents, records, or specimens) that have been collected, or will be collected solely for non-research purposes (such as medical treatment or diagnosis)
Research involving materials (data, documents, records, or specimens) that have been collected, or will be collected solely for non-research purposes.
-
Category 6 - Collection of data from voice, video, digital, or image recordings made for research purposes
Collection of data from voice, video, digital, or image recordings made for research purposes.
-
Category 7 - Research on individual or group characteristics or behavior
Research on individual or group characteristics or behavior employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies.
NOTE: Some research in this category may be exempt from the HHS regulations for the protection of human subjects. 45 CFR 46.101(b)(4). This listing refers only to research that is not exempt.
-
Category 8 - Continuing review of research previously approved by the convened IRB
Continuing review of research previously approved by the convened IRB but is not active in data collection and the research activities are limited to data analysis.
-
Category 9 - Continuing review of research, not conducted under an investigational new drug application or investigational device exemption
Continuing review of research that involves no greater than minimal risk and no additional risks have been identified.
-
-
-
Full Board Review
-
Information
A Full Board Review is required for research that is not eligible for exempt or expedited review. In short, research that is judged to involve more than minimal risk or involves protected populations, such as children, prisoners, or disabled individuals, must undergo a full board review. Individuals intending to conduct research that requires a full board review should allow ample time to complete the review process.
-
Greater than minimal risk
Projects for which the level of risk is determined and verified by the IRB Chair to be greater than minimal such as projects that plan to use procedures that are personally intrusive, stressful, or potentially traumatic (stress can be physical, psychological, social, financial, or legal).
-
Deception
Projects that involve the intentional deception of subjects, such that misleading or untruthful information will be provided to participants.
-
Vulnerable Population
Projects that involve sensitive or protected populations (such as children, pregnant women prisoners or cognitively disabled individuals).
-